Vyalev: A Newly-Approved Medication for Parkinson’s Disease Motor Fluctuations
Breaking news! The first subcutaneous (under the skin) 24-hour infusion of foslevodopa/foscarbidopa under the brand name Vyalev, was approved by the Food and Drug Administration on Oct 17, 2024. Produced by AbbVie, this medication for the treatment of motor fluctuations in Parkinson’s disease (PD) has already been approved in many countries and is now available for people with PD in the US. Foslevodopa/foscarbidopa is a soluble form of carbidopa/levodopa which is quickly converted into carbidopa/levodopa and can lead to steady blood levels of levodopa within 24 hours.
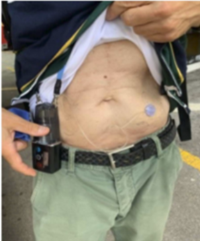
The medication is infused via a subcutaneous pump which is shown here. This is a non-surgical regimen that provides a continuous delivery of levodopa.
The FDA based their approval on a clinical trial in which after 12 weeks of treatment, patients receiving foscarbidopa/foslevodopa showed a significantly greater decrease in OFF time (mean reduction of -1.79 hours) and an increase in ON time without troublesome dyskinesias (mean reduction of 1.75 hours) compared with the group receiving immediate-release carbidopa/levodopa.
While carbidopa-levodopa is not a new medication, new formulations of it give people with Parkinson’s disease more options to customize treatment and increase quality of life.
It is early days for implementing this new treatment and the processes for getting started are unfolding. Insurance coverage, including Medicare coverage will evolve over the next few months as well. Vyalev may be most appropriate for those with advanced PD, but if you are having trouble motor fluctuations, speak with your doctor about this new option, as well as about Crexont, a new extended-release oral formulation which has recently been approved as well.